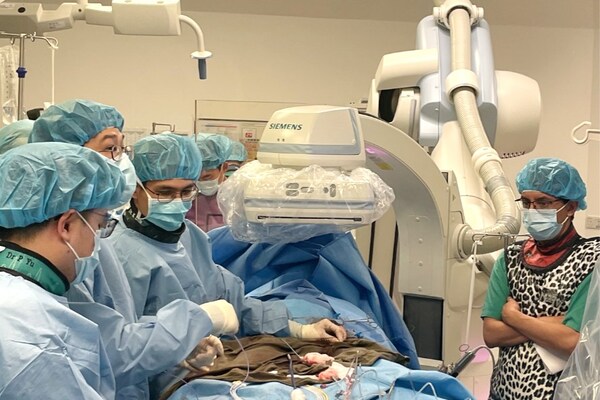
HANGZHOU, China, Sept. 5, 2024 /PRNewswire/ -- Venus Medtech (Hangzhou) Inc. (Venus Medtech, 02500.HK), a leading provider of integrated solutions for transcatheter structural heart valvular therapies in China, announced that its in-house developed next-generation balloon-expandable dry-tissue transcatheter aortic valve replacement (TAVR) system, Venus-Vitae, successfully completed two inaugural implantations as part of its global multicenter pivotal trial, Venus-Vitae SMART-ALIGN, at Prince of Wales Hospital in Hong Kong, China.
These successful implantations mark another leap forward for the company into the frontier of next-generation dry-tissue valves, representing a significant addition to our transcatheter heart valve solutions.
Following the first two successful implantations, Venus-Vitae SMART-ALIGN pivotal clinical trial will be progressively launched at around 20 centers in Europe, North America and China, enrolling a total of 150 patients. The trial data will be used to support registration applications in the EU, Canada, China and other territories.
The procedures were performed by Prof. So Chak Yu Kent and the team at Prince of Wales Hospital in collaboration with Prof. Scott Lim of University of Virginia Medical Center, supported by a multidisciplinary team from Ultrasound, Anesthesiology, and Imaging Departments. Both patients received 23mm Venus-Vitae implants. The procedures presented good immediate post-operative results with significant hemodynamic improvement.
Venus-Vitae Smart-ALIGN, abbreviated from Multicenter Pivotal Study to Evaluate the TreatMent of Severe AoRTic VALve StenosIs UsinG the VeNus-Vitae Transcatheter Heart Valve System, is a prospective, multicenter, non-randomized interventional study to evaluate the safety and efficacy of the Venus-Vitae TAVR system in patients with severe aortic stenosis.
"Recently, my colleague Dr. Michael Hainstock and I, at the University of Virginia Medical Center, completed the first patient implantation in the VenusP-Valve PROTEUS pivotal clinical trial in the United States, " said Prof. Scott Lim." Two months later, I am very pleased to have participated with Dr. Kent So in the implantation of the first two patients in the Venus-Vitae SMART-ALIGN international multicenter pivotal clinical trial at Prince of Wales Hospital in Hong Kong. These both represent important milestones in those studies. Additionally, both the VenusP Valve and the Vitae valve have been previously used in clinical applications, with excellent published results. Of note, the Venus-Vitae valve's stent frame is the shortest among current transcatheter aortic valves, and I am encouraged by its potential to treat aortic stenosis while maintaining coronary access. Furthermore, its dry valve leaflet technology facilitated rapid preoperative preparation time. We look forward to further international enrollment in the SMART-ALIGN clinical trial in the near future."
"It was an honor to collaborate with Dr. Scott Lim to complete the first two confirmatory clinical implantations of this innovative dry valve," said Prof. So Chak Yu Kent after the procedures. "Venus-Vitae's active anti-PVL technology and short frame design effectively prevent leakage and minimize the risk of coronary artery obstruction. I'm eager to see expedited enrollment of patients for Venus-Vitae, so that more patients can benefit soon."
"Venus-Vitae is our next generation balloon-expandable TAVR system, with global patent coverage," said Hou-Sen Lim, General Manager and CEO of Venus Medtech. "We have strong confidence in its international multi-center clinical trials and global strategy. The successful initial implantations provide a solid foundation for forthcoming trials in Europe, North America, and China. We are committed to advancing Vitae's global clinical application and regulatory registration, bringing our cutting-edge technology to physicians and patients worldwide."
About Venus-Vitae
Venus-Vitae utilizes the innovative Venus-Endura technology, which integrates multiple anti-calcification techniques, immunogenicity removal technology and 3D force-controlled dehydration technology, providing exceptional durability, biocompatibility, and excellent anti-calcification performance. In addition, this design allows for room temperature storage of the valve in a dry-tissue condition.
Additionally, the product features a unique patented technology, Lockwire Technology, ensuring the stability and accuracy of the valve during delivery and deployment. The short stent frame of Venus-Vitae's valve along with its steerable NAVIMASTER delivery system, significantly optimizes the performance across the aortic arch and positioning within the native valve. Venus-Vitae also features a unique Coronary Alignment technology, addressing the fundamental challenge that current TAVR products have been unable to address—aiding in protection of the coronary arteries. By utilizing three gold markers at the base of the valve leaflets in conjunction with the delivery system's steering, telescoping, and balloon rotation capabilities, Venus-Vitae can effortlessly and precisely achieve coronary alignment during the deployment process. This aids in accomplishing coronary protection throughout the implantation procedure.
Furthermore, Venus-Vitae features a self-adaptive anti-PVL technology, utilizing an adaptive polymer skirt. The proprietary polymer skirt material possesses a high compression ratio, excellent resilience, self-expanding properties, and adaptive sealing. It demonstrates adaptive deformation during valve crimping, without enlarging the profile of the delivery system. Upon deployment, it actively fills the gaps around the valve perimeter, achieving remarkable anti-PVL performance.
About Venus Medtech
Venus Medtech (Hangzhou) Inc. (02500.HK) is committed to structural heart innovation. We are developing and commercializing comprehensive solutions for structural heart disease. Our robust pipeline, encompassing all four heart valves from TAVR, TPVR, TMVR, and TTVR to hypertensive renal denervation (RDN) therapy, underscores our unwavering commitment.
For more information, please visit https://www.venusmedtech.com
*Provided for informational and academic purposes only, this content is not intended as professional medical or legal advice. Venus Medtech makes no representations, warranties or guarantees regarding the completeness, accuracy, or timeliness of this content.
*Venus Medtech makes no representations, warranties or guarantees regarding the property or clinical performance of any medical devices mentioned.
*VENUSMEDTECH, the stylized QI logo, Venus-Vitae, Venus-Endura etc. are trademarks of Venus Medtech (Hangzhou) Inc.
*Venus-Vitae is currently restricted to research only in the EU, North America, and China.
source: Venus Medtech (Hangzhou) Inc.
【與拍賣官看藝術】走進Sotheby's Maison睇睇蘇富比旗艦藝廊!蘇富比如何突破傳統成規?► 即睇